The geometry of the BeCl2 molecule can then be predicted using the Valence Shell Electron Pair Repulsion Theory VSEPR Theory which states that molecules will choose a BeCl2 geometrical shape in which the electrons have from one another. Beryllium dichloride is a compound of beryllium 2 oxidation state and chloride in the ratio 12.
Solved Exercise Draw The Lewis Structure Os 1 Becl2 2 Chegg Com
Bcl3 Vsepr Shape Becl2 Lewis Structure How To Draw The Lewis Structure Find Out By Adding Single Double Or Triple Bonds And Lone Pairs To The Central Atom Victoria Schlink
Nanopdf Com
It doesnt have 4 valence electrons the reason being is because the bonds between beryllium and chlorine are ionic which means its transferring giving up its two electrons opposed to sharing them one to each chlorine atom.

Becl2 lewis structure. See full answer below. This is the lewis structure for BeCl2 Be has 2 valence electrons which are shared by covalent bonding. Beryllium chloride is a white to green solid with a sharp odor.
Lewis structure of BeCl2. Note that a contains P which is a Period-3 element and can have an expanded valence shell. Thus Be is neutral.
It is a beryllium molecular entity and an inorganic chloride. BeCl2 Lewis Structure. BeCl2 exists as a discrete molecule.
The Lewis structure of any given molecule helps to know the arrangement. Below are the valence orbitals of ceBeCl2 DF-BP86def2-SVP. Include all lone pairs of electrons.
And these are only depicted in the right hand side structure above. These electrons will be both bonding as well as non-bonding electrons. 5 rows BeCl2 Lewis Structure.
The unbonded sides of the Cl atoms then. Formal charge on Be is zero. The Lewis structure of BeCl 2 is.
Drawing BeCl2 Lewis Structure is very easy. On the left and right sides are a single bond attached to a Cl atom. 7 rows BeCl2 Lewis Structure.
What other resonance structures are possible that satisfy the octet rule Draw the molecule by placing atoms on the grid and connecting them with bonds. The BeCl2 Lewis structure is a diagram that illustrates the number of valence electrons and bond electron pairs in the BeCl2 molecule. Beryllium forms single covalent bonds through both the Chlorine atoms.
Pick the most likely structure. BeCl2 contains 2 bonding pairs of electrons which arrange themselves as far away as possible to minimise the electron repulsion between them giving a bond angle of 180 degrees and a. Beryllium Dichloride on Wikipedia.
Click and drag the molecle to rotate it. Lewis structure of the molecule is based on the concept of the octet rule. Beryllium Be doesnt need 8 valence electrons to have an octet Be often only needs 4.
To change the symbol of an atom double. Here in this post we described step by step method to construct BeCl2 Lewis Structure. The electrons present in the outermost shell of an atom are shown in the Lewis structure of any molecule.
On the left and right sides are a single bond attached to a Cl atom. This bonded pairs of electrons take the location as much as possible to stop the repulsive forces resulting in 180 link angle. The beryllium hydride molecule consists of one beryllium atom and two hydrogen atoms.
Each chlorine atom contributes 7 valence electrons and the lone beryllium atom contributes 2 valence. The Lewis structure of BeCl2 is. B BFCl 2.
The resonance is formed when an electron pair from each chlorine atom forms a double bond with Be. The electrons present in the outermost shell of an atom are shown in the. Lewis structure of BeCl2.
What is Lewis structure of BeCl2. If youre not sure you have the best Lewis structure for BeCl 2 you can calculate the formal charges. BeCl2 has straightforward structure and molecular geometry.
Hence total number of valence electrons in beryllium hydride are 2 1 x 2 4 electrons. Barium chloride - YouTube. The number of electrons on the valence shell of Be and Cl is 2 and 7 electrons respectively.
The BeCl 2 Lewis structure is similar to BeF 2 since F is in Group 7 and has 7 valence electrons. Therefore this molecule is nonpolar. Hence all the valence electrons are used up and there are no lone pairs of electrons in this molecule.
There is neither deficiency nor excess of electrons with Be in the lewis structure. USCG 1999 CAMEO Chemicals. The molecular geometry of BeCl 2 is linear with symmetric charge distribution around the central atom.
It has a role as a carcinogenic agent and a genotoxin. For this dot structure start with the Be atom in the center. How to Draw the Lewis Dot Structure for BaCl2.
A H 3 PO 4 has two resonance forms and formal charges indicate the more. The electronic configuration of beryllium is 2s2and chlorine is 3s23p5. So you can see that one Lewis structure is not enough to actually describe the bonding in that molecule well but as a first approximation the left hand structure above should be sufficient.
For this dot structure start with the Be atom in the center. A step-by-step explanation of how to draw the BeCl2 Lewis Dot Structure Beryllium DichlorideBeryllium Be is a bit of an exception and is okay with only. Resonance structures are used to represent the different possible bonding arrangements in a molecule.
Draw the Lewis structures for the molecule and determine if there is an element which can be an exception to the octet rule.
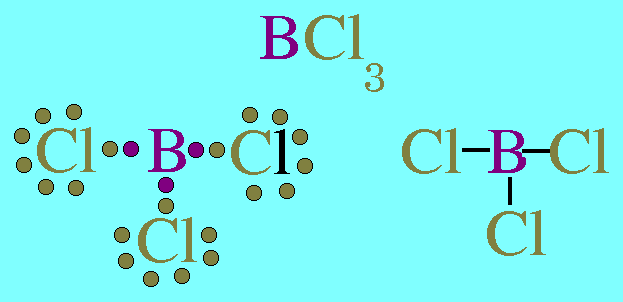
How Many Electrons Are In The Valence Shells Of A Be In Becl2 B B In Bcl3 C H In H2o Socratic

Which Lewis Structure For Becl2 Is More Commonly Seen Chemistry Stack Exchange

Becl2 Lewis Structure And Molecular Geometry Youtube
Chemist Sg
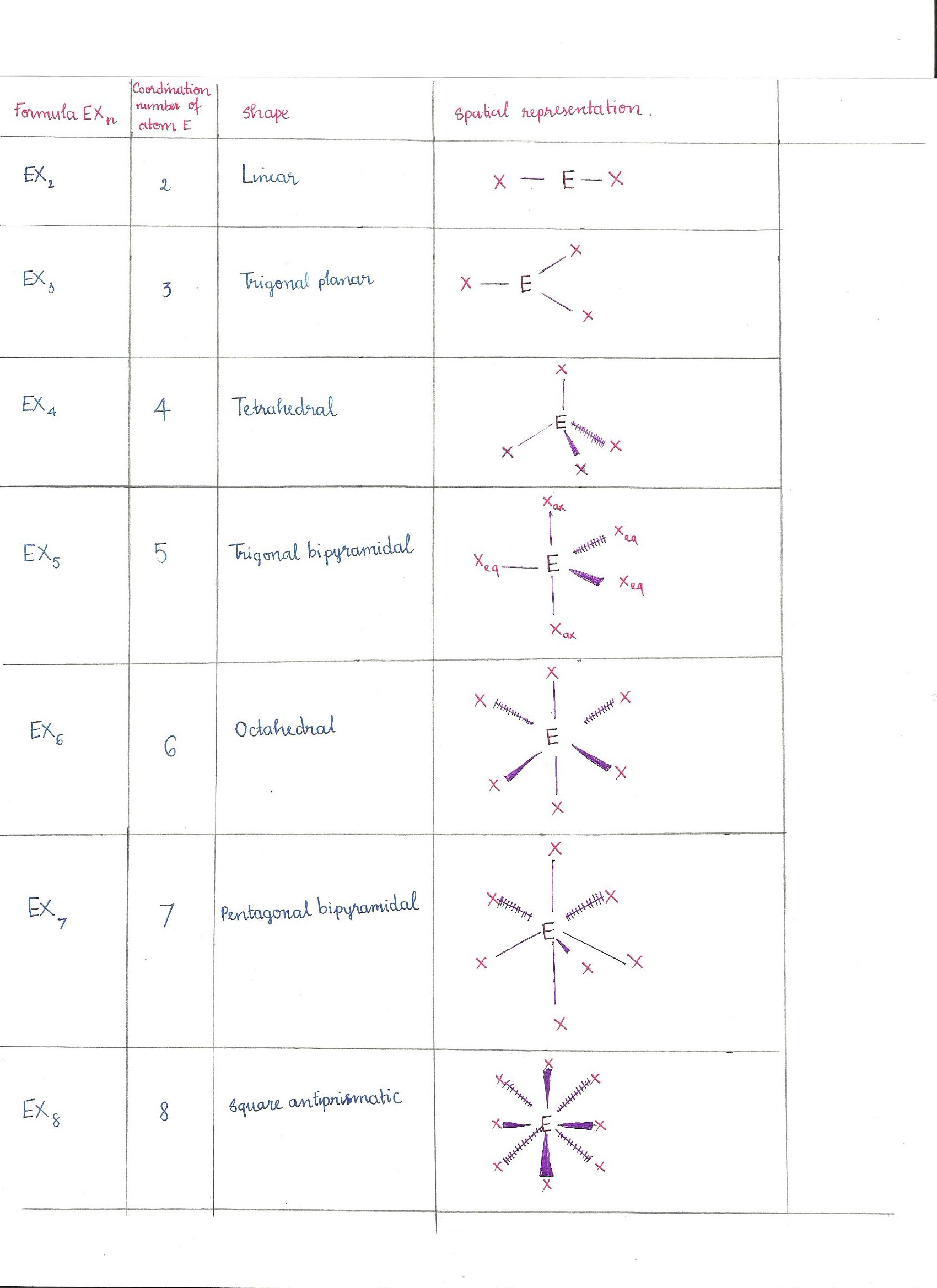
Vsepr Chemistry Libretexts

Becl2 Polar Or Nonpolar How To Discuss
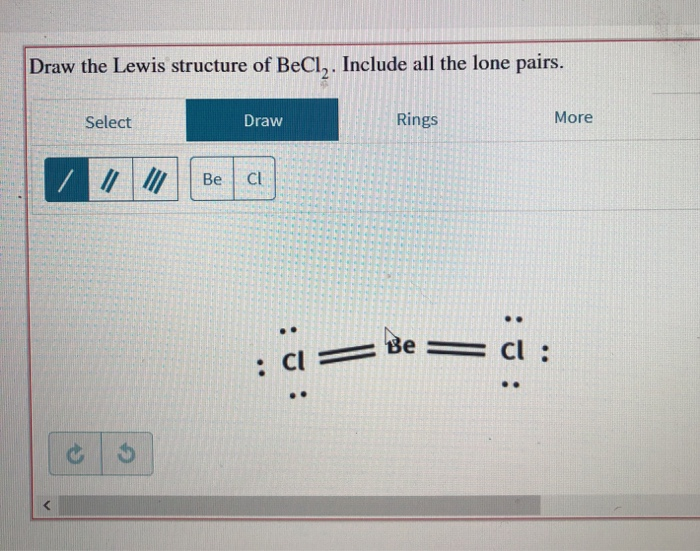
Solved Draw The Lewis Structure Of Becl Include All The Chegg Com
What Is The Formal Charge Of Becl2 Quora